TOC – OCD – TDCs para el trastorno obsesivo compulsivo.
Protocol for Transcranial Direct Current Stimulation for Obsessive-Compulsive Disorder
https://www.mdpi.com/2076-3425/10/12/1008
Open AccessArticle
Protocol for Transcranial Direct Current Stimulation for Obsessive-Compulsive Disorder
by
* ,
,
and
School of Psychology in Faculty of Health Sciences, Curtin University, Kent Street, Bentley, WA 6102, Australia
*
Author to whom correspondence should be addressed.
Submission received: 13 October 2020 / Revised: 9 December 2020 / Accepted: 15 December 2020 / Published: 18 December 2020
Abstract
Obsessive-compulsive disorder (OCD) is a debilitating disorder with an approximate lifetime prevalence of 1–3%. Despite advances in leading treatment modalities, including pharmacotherapy and psychotherapy, some cases remain treatment resistant. Non-invasive brain stimulation has been explored in this treatment-resistant population with some promising findings; however, a lack of methodological rigor has reduced the quality of the findings. The current paper presents the protocol for conducting research into the efficacy of transcranial direct current stimulation (tDCS) in the treatment of OCD. A double-blind randomised controlled trial (RCT) will be conducted involving active tDCS vs. sham tDCS on 40 general OCD patients. The intervention consists of 2 mA anodal stimulation over the pre-supplementary motor area (pre-SMA) with the cathode positioned over the orbitofrontal cortex (OFC). Participants will receive 10 sessions of 20 min of either sham- or active-tDCS over 4 weeks. Outcomes will be categorical and dimensional measures of OCD, as well as related secondary clinical measures (depression, anxiety, quality of life), and neurocognitive functions (inhibitory control, cognitive flexibility).
Participants will be seated in a comfortable armchair whilst receiving tDCS, which will be delivered for 20 min using a battery-driven (Necbox) multi-channel direct current stimulator, namely Starstim 20TM (Neuroelectrics, Barcelona, Spain). Stimulation will be administered over the scalp via two 25 cm2 Sponstim electrodes (Neuroelectrics, Barcelona, Spain). The electrodes consist of a sponge cover, a carbon rubber core, and a nickel-plated brass metallic pin. The external surface of the sponges will be soaked in 5 mL of 0.9% saline solution to minimise the risk of skin irritation, and inserted into Fz (pre-SMA) and Fp1 (OFC) of an adult-sized neoprene cap (S, M, or L), which is pre-labelled according to the 10–20 EEG system of electrode positioning. The participant’s head will be measured to locate Cz (mid-line central part of the head), and then the cap will be placed on the participant’s head. Once the cap is in the correct position (with Cz lined up), it will be secured in place with a chin strap, and the medical sockets will be connected to the electrodes. An impedance check will be conducted to ensure optimal conductivity. If the impedance level is high, more saline solution will be added onto the surface of the sponges by inserting a curved syringe through a hole in the cap near the electrode.
Each session will involve 20 min of either active or sham stimulation. The tDCS montage (stimulation site, intensity, duration, and frequency) is informed by Kekic’s 2016 systematic review [
21]. The participants in the active group will receive a constant current 2 mA stimulation via the anode placed over the pre-SMA and cathode placed over the left OFC. The anode will increase neural activation of the pre-SMA and the cathode will decrease neural activation of the left OFC (). For the sham stimulation, the electrode montage will be identical to the active tDCS group; however, the participants will only receive tDCS for 30 s at the start, ramp up (0–2 mA) and end, ramp down (2–0 mA) of the session. This allows the participants to experience some sensation of tDCS. All participants (active and sham) will be informed that they may or may not perceive any sensation during the treatment, a procedure that has been demonstrated to effectively blind participants and the researcher to the stimulation condition they are in [
22].
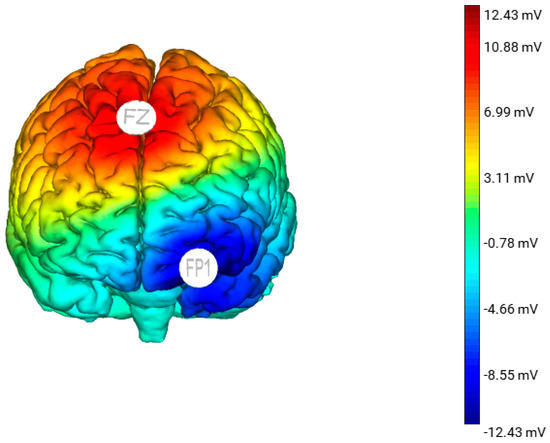
Figure 1. A 3D standardised model of the estimated electric field generated from anodal stimulation over Fz with the cathode placed over Fp1. This model was produced using the Neuroelectric Stim 20TM Preview function.
3.1. Outcome Measures
Diagnostic screening, neuropsychological, and cognitive assessment measures for this study will be administered at baseline, post-intervention, 3-month-, and 6-month follow-up. The Y-BOCS, Depression Anxiety Stress Scales (DASS-21), and Obsessive Beliefs Questionnaire (OBQ-44) will also be administered at the end of the 1st, 2nd, and 3rd week of the intervention period to identify if, and when, changes may occur. OCD symptom change, as measured by the Y-BOCS, is the primary outcome being assessed in this study.
Diagnostic screening: The MINI-7.0.0 [
20] will be used for clinical diagnosis of OCD.
OCD symptom severity: The Y-BOCS [
23] measures symptom type and severity over the last 7-day period and consists of two 10-item subscales, namely obsessions and compulsions.
Negative affect: The Depression Anxiety Stress Scales (DASS-21) [
24] is a self-report measure to identify and measure negative affect (if present).
Quality of life: The Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q-SF) [
25], also a self-report measure, has 16 items evaluating overall enjoyment and satisfaction with physical health, mood, work, household and leisure activities, social and family relationships, daily functioning, sexual life, economic status, overall well-being, and medications.
OCD beliefs: The Obsessive Beliefs Questionnaire (OBQ-44) measures OCD beliefs. The OBQ-44 includes (1) perfectionism and intolerance of uncertainty, (2) importance and control of thoughts, (3) responsibility, and (4) overestimation of threat, which are positively associated with obsessive-compulsive symptoms and worry [
26].
Inhibitory function: The Stop Signal Task is a choice go/no-go reaction-time task to measure inhibitory control. In this computerised task, participants are required to discriminate between left and right arrows by pressing the appropriate response key as fast as possible (go), but inhibit their motor response if a beep is played after the presentation of the arrow (no-go) [
27]. The task is designed so that the frequency of the ‘go’ cues are greater than the ‘no-go’ cues, resulting in the ‘go’ response becoming prepotent and thus control being required to inhibit/withhold the response [
28].
Cognitive flexibility: The set-shifting task will be used to measure cognitive flexibility. Set-shifting measures the ability to shift attention and respond to a particular aspect of a stimulus depending on a reinforced contingency. The rules or contingencies of the task change and alternate rapidly, requiring the participant to pay attention and respond with the pertinent rule in mind, switching from the old to the new rule. Individuals with OCD demonstrate repetitive and perseverate behaviour, and impairments in set-shifting ability have been reported to be a key neurocognitive feature of OCD [
28,
29].
3.2. Potential Side Effects
The procedure is considered very low risk, and no significant adverse events have been reported with low-current procedures such as the ones proposed in this study. Potential side effects of low-current tDCS include localised scalp itching or tingling sensation at the site where the electrode was placed, and seldom-occurring headache or fatigue [
30]. If any of these side effects occur, the participant will be monitored; if they do not dissipate within 1 hour (the typical duration of symptoms/s), the participant will be referred for assessment by a medical practitioner. Any adverse side effects will be reported to the appropriate ethics committee. Discontinuation and/or withdrawal from the study will be recorded in the study database.
Transcranial Direct Current Stimulation Improved OCD in Controlled Trial
Shavitt and colleagues gave 30 minutes of either active or sham tDCS for 20 days to patients with treatment-resistant OCD. They positioned the cathode over the supplementary motor area of the brain, and the anode over the left deltoid. Those patients who received active tDCS achieved significantly greater reductions in OCD symptoms than did those in the sham group.
Transcranial direct current stimulation in patients with obsessive compulsive disorder: A randomized controlled trial
https://www.cambridge.org/core/journals/european-psychiatry/article/transcranial-direct-current-stimulation-in-patients-with-obsessive-compulsive-disorder-a-randomized-controlled-trial/609630E6B0C14EB3109DB9A307BFC56F
2.2 tDCS parameters
Stimulation sessions were delivered using a neuroConn DC stimulator (Ilmeneau, GmbH) with two 7 × 5 cm (35 cm2) sponge electrodes soaked in a saline solution (0.9% NaCl). The patients received 2 sessions of tDCS per day for 5 consecutive days (from Monday to Friday). The two daily sessions were separated by at least 3 h. Each session of active tDCS consisted of delivering a direct current with an intensity set at 2 mA for 20 min. The electrodes were placed according to the international 10–20 electrode placement system. The cathode was placed on FP1 to target the left OFC, and the anode was placed 3 cm below the inion and 1 cm right of the midline to target the right cerebellum. To maintain blinding of the experimenters and participants regarding the tDCS condition, we used the commercial built-in sham procedure of the device (double-blind ‘study mode’). The care provider entered a preprogrammed code that delivered either active or sham tDCS but was unaware of which condition the code applied. The list of codes was established by a researcher not involved in the tDCS delivery, data collection or analyses. In the sham, the same stimulation parameters as in the real condition were displayed, but after 40 s of real stimulation (2 mA), only brief current pulses of 110 μA over 15 ms occurred every 550 ms through the remainder of the 20-min period. Participants, care providers, investigators, and outcome assessors were blinded to the treatment assignment. Safety was assessed after each tDCS session with a structured interview [Reference Brunoni, Amadera, Berbel, Volz, Rizzerio and Fregni33], and serious adverse events were systematically recorded by the investigator.
2.3 Clinical outcomes
All clinical outcomes were measured at baseline (before the first tDCS session), immediately after the 10 tDCS sessions (M0), and one month (M1) and three months after tDCS (M3).
Obsessive and compulsive symptoms were assessed using the Y-BOCS, the clinical global impression scale (CGI) [Reference William34] and a self-reporting OCD visual analogue scale (OCD-VAS). To complete the OCD-VAS, patients were required to rate their current obsessive and compulsive symptoms on a 10-point scale (0 = Worst Ever to 10 = Best Ever). The clinical response was defined as a decrease ≥35% on the Y-BOCS or a score of 2 or less on the CGI-I (much or very much improved). Depressive symptoms and anxiety were assessed using the MADRS [Reference Montgomery and Asberg32] and State-Trait Anxiety Inventory (STAI-YA) [Reference Spielberger35], respectively. Level of functioning was assessed by the Global Assessment of Functioning (GAF) scale [Reference Pedersen, Urnes, Hummelen, Wilberg and Kvarstein36].
2.4 Statistical analysis
Statistical analyses were performed using SPSS (version 22). Fisher’s exact tests and two-tailed Student’s t-tests were applied to compare the demographics and clinical measures at baseline between the active and sham groups for categorical and continuous variables, respectively.
The primary analysis investigated acute changes in the YBOCS score. Acute changes in clinical scores between baseline and after the 10 tDCS sessions (at M0) were compared using repeated-measures analysis of variance (ANOVA) with treatment as the intergroup factor and time as the intrasubject factor. For significant changes, post hoc comparisons were performed using Fisher’s least significant difference tests.
Secondary clinical analyses investigated maintenance effects on the YBOCS score, response rate and effects on other symptoms. Changes in the YBOCS scores over the 12-week follow-up period were compared using repeated-measures analysis of variance (ANOVA), including terms for group, time, and time by group interaction. Response rates at each time point (defined as an Y-BOCS score reduction of at least 35% between inclusion and month 1 post-tDCS) were compared using Fisher’s exact test. The effects on other symptoms (MADRS, CGI, STAI, VAS) were analyzed in the same manner.
All tests were conducted with two-sided significance levels (α = 0.05).
3. Results
3.1 Demographics and baseline clinical characteristics
Twenty-one patients were recruited and completed the trial. As shown in Table 1, the active and sham groups did not differ significantly in demographics or baseline clinical ratings.
Table 1 Demographic and clinical baseline characteristics of the patients with OCD included in the study. No differences were observed between patients in the active and sham groups. The results are given as the mean (SD: standard deviation).